Abstract
Introduction: The use of combined rituximab (R)-bendamustine(B) has shown to be effective in terms of overall response rate (ORR), progression-free survival (PFS) and overall survival (OS) in pts with relapsed/refractory CLL. Based on the enhanced in vitro and in vivo anti-CLL activity of obinutuzumab compared to rituximab, we designed this phase II study to evaluate the efficacy of obinutuzumab(Obi) with B in patients (pts) with relapsed or refractory CLL.
Methods Between April 2014 and April 2017, 72 adult pts with relapsed or refractory CD20+ CLL (according to NCI criteria) and active disease (IWCLL 2008) were included in this prospective, multicenter, non-comparative, phase II study. Pts received up to six-28-day cycles of treatment consisting of obinutuzumab (1000 mg) given by intravenous (IV) infusion on Days (D) 1, 8, and 15 of Cycle 1 and on D1 of subsequent cycles, and B (70 mg/m2) by IV infusion on D2 and 3 of Cycle 1 and on D1 and 2 of subsequent cycles. The primary endpoint was ORR (by IWCLL 2008); secondary endpoints included best RR, OS and PFS, duration of response and minimal residual disease (MRD) rate detected by multicolor flow cytometry (sensitivity: 1 CLL cell in 10-4). We present the results of an interim analysis at 6 months of last Obi-B cycle with the data cut-off date of 25th of April of 2017.
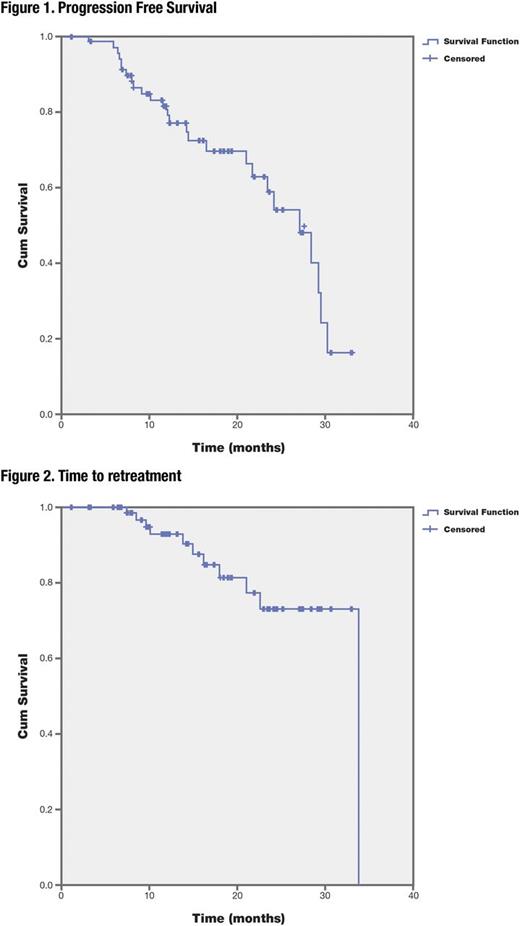
Results. Pts were mostly male (66.7%), with a median age of 67.9 (61.6-72.7) years and ECOG 0 in 62.5% of them. Rai clinical stage was low/intermediate (0-II) in 52.8% pts and high-risk (III-IV) in 47.2% pts. Forty eight pts relapsed after an FC+/-R (58.3%) or B-R (8.3%) therapy and 7(9.7%) pts were refractory to last immunochemotherapy. Expression of CD38 (>30%), CD49D (>20%), ZAP-70 was seen in 15.3%, 55.6%, and 39.4% of pts respectively, and unmutated immunoglobulin heavy chain gene (IGHV) status in 75.0% of pts. Genetic abnormalities detected by FISH and Sanger sequencing were: del13q(44.4%), trisomy 12(18.05%), del11q/ATMmutation(43.05%), del17p/p53mutation (26.3%), mutations of BIRC3(4.1%), SF3B1(9.7%) and NOTCH1(23.6%). Out of 72 enrolled pts, 80.6% and 79.2% completed 6 cycles of treatment with Obi and B respectively. Median (range) treatment duration was 4.9 (4.6-5.1) months. Obi doses were modified in 75% of pts (premature discontinuations: 4%; infusion rate reduction: 15.9%; temporarily interruptions: 77.8%). B was modified in 52.8% of pts (premature discontinuations: 7.2%; temporarily interruptions: 55.4%; dose reductions: 30.1; dose reduction rate: 2.4%). On an intent to treat analysis, 70(97.2%) pts were evaluable for efficacy according to protocol, the ORR was 81.4 % (CR 35.7%, CRi 7.1% and PR 38.6%), PD (2.9%) and SD (5.7%). In the 7 pts with refractory CLL, the ORR was 71.4% (CR 42.9%, CRi 14.3% and PR 14.3%) and SD 28.6%. After a median follow-up of 13.7 months, the median PFS was 27.0 months (figure 1). The median time to retreatment 33.8 months (figure 2). Significant differences were found in the PFS according to MRD status in Bone Marrow (Category: Cat.0: MRD level<0.01, 30.2 months, Cat. 1: MRD between 0.01-0.1: 21.6 months and Cat. 2:MRD>0.1: 12.1 months; p<0.001) and in the PFS regarding the IGHV mutation status (unmutated, 24.1 months vs mutated, median non-reached; p<0.05). The presence of p53 and ATM disruption did not have a significant impact on response, perhaps because of the high RR (81.4%). Sixty-three pts (87.5%) reported at least 1 toxicity, being the most common grade 3/4 neutropenia (51.4%) and thrombocytopenia (19.5%). In total, 82 SAEs or AESI were reported in 34(53.9%) pts. The discontinuation rate due to AEs was 18.1%. Two deaths occurred due to toxicities, acute myeloid leukemia and septic shock.
Conclusions This interim analysis anticipates that the combination of obinutuzumab with Bendamustine is feasible, effective with an ORR of 81.4% and manageable in relapsed/refractory CLL. Achievement of MRD negativity in the bone marrow of pts treated with Obinutuzumab+Bendamustine seems associated with a longer PFS regardless of genetic abnormalities.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal